This web page was produced as an assignment for Genetics 677, an undergraduate course at UW-Madison
Conclusions
Narcolepsy is a chronic sleep disorder characterized by excessive daytime sleepiness. Individuals with narcolepsy experience several other debilitating symptoms including cataplexy, sleep paralysis, and hallucinations. An estimated 3 million people are affected by narcolepsy, however either the cases go undiagnosed or the symptoms are mistaken for depression, epilepsy, the side effects of drugs, or just being lazy. Onset occurs around adolescence, but there is usually a delay of 15 years between onset and diagnosis. Medications exist to treat the symptoms, but no known cure exists as the cause of narcolepsy is not fully understood. Stimulants exist to combat daytime sleepiness, however most are extremely addictive such as amphetamines. Anti-depressants are used to treat cataplexy, hallucinations, and sleep paralysis. Even with these medications, many individuals affected with narcolepsy cannot live a completely normal life because of the frequent naps they must take, the danger of sudden paralysis brought on by feeling strong emotions, and the embarrassment of falling asleep at inappropriate times.
While there is no known cause, there is a known condition associated with the disorder. Individuals affected with narcolepsy are known to have extremely low amounts of the neuropeptide hypocretin in the body. Hypocretin is produced by neurons in the lateral hypothalamus and these neurons project into many areas of the brain. They have been shown to have an important role stabilizing wakefulness and sleep, and in inducing appetite. While there is no known cause in humans, the cause for canine narcolepsy is a mutation in the hcrtr1 gene which encodes the hypocretin receptor 1 protein. Therefore, I dedicated this website to learning all I could about this particular gene.
I learned many things about hcrtr1 over the semester. Some of this information that I found most interesting was the size of the hypocretin receptor 1 protein is only 425 amino acids long, that it has one protein domain in it - the 7 transmembrane G-protein coupled receptor, that this family of proteins is very large and plays many important roles in signaling pathways all over the body, and that while there are no known homologs in C. elegans or Drosophila, there are highly conserved hcrtr1 homologs in several other model organisms including mice. While all of this information was helpful in understanding the function of hcrtr1 in narcolepsy, I think the protein interactome information has the greatest implication for further research.
Using the protein interaction website STRING, I learned that the hypocretin receptor 1 interacts with the cannabinoid receptor 1 protein, so I decided to look further into that interaction. The cannabinoid receptor 1 protein shows many similarities to the hypocretin receptor 1 protein: both are G-protein coupled receptor proteins, both are located in the lateral hypothalamus, and both have a function in promoting food intake. The interaction between these similar receptors referenced a study in which activating the cannabinoid receptor 1 had a dramatic increase in the hypocretin receptor 1 response. Armed with this information, I decided upon an experiment to see if this could have some impact in treating narcolepsy.
I would like to perform an experiment in which I used hypocretin KO mice to test the interaction of the two receptor proteins and see if their location in an actual organism is in a close enough proximity for the cannabinoid receptor 1 to induce a high hypocretin 1 response as demonstrated in the study. The study did not test this interaction in vivo. Additionally, it co-expressed the two genes. Therefore, I would like to see if this interaction is feasible in treating narcolepsy symptoms in organisms. Mice were shown to have high homology for the hypocretin 1 receptor, and it has been proven that hypocretin KO mice have similar symptoms to narcoleptic humans (1). If activation of the cannabinoid receptor 1 by exposing mice to synthetic cannabinoids induces a high hypocretin receptor 1 response, then maybe these narcoleptic mice will no longer suffer the narcoleptic symptoms! I could confirm the interaction between these two proteins with a yeast-2 hybrid technique.
While there is no known cause, there is a known condition associated with the disorder. Individuals affected with narcolepsy are known to have extremely low amounts of the neuropeptide hypocretin in the body. Hypocretin is produced by neurons in the lateral hypothalamus and these neurons project into many areas of the brain. They have been shown to have an important role stabilizing wakefulness and sleep, and in inducing appetite. While there is no known cause in humans, the cause for canine narcolepsy is a mutation in the hcrtr1 gene which encodes the hypocretin receptor 1 protein. Therefore, I dedicated this website to learning all I could about this particular gene.
I learned many things about hcrtr1 over the semester. Some of this information that I found most interesting was the size of the hypocretin receptor 1 protein is only 425 amino acids long, that it has one protein domain in it - the 7 transmembrane G-protein coupled receptor, that this family of proteins is very large and plays many important roles in signaling pathways all over the body, and that while there are no known homologs in C. elegans or Drosophila, there are highly conserved hcrtr1 homologs in several other model organisms including mice. While all of this information was helpful in understanding the function of hcrtr1 in narcolepsy, I think the protein interactome information has the greatest implication for further research.
Using the protein interaction website STRING, I learned that the hypocretin receptor 1 interacts with the cannabinoid receptor 1 protein, so I decided to look further into that interaction. The cannabinoid receptor 1 protein shows many similarities to the hypocretin receptor 1 protein: both are G-protein coupled receptor proteins, both are located in the lateral hypothalamus, and both have a function in promoting food intake. The interaction between these similar receptors referenced a study in which activating the cannabinoid receptor 1 had a dramatic increase in the hypocretin receptor 1 response. Armed with this information, I decided upon an experiment to see if this could have some impact in treating narcolepsy.
I would like to perform an experiment in which I used hypocretin KO mice to test the interaction of the two receptor proteins and see if their location in an actual organism is in a close enough proximity for the cannabinoid receptor 1 to induce a high hypocretin 1 response as demonstrated in the study. The study did not test this interaction in vivo. Additionally, it co-expressed the two genes. Therefore, I would like to see if this interaction is feasible in treating narcolepsy symptoms in organisms. Mice were shown to have high homology for the hypocretin 1 receptor, and it has been proven that hypocretin KO mice have similar symptoms to narcoleptic humans (1). If activation of the cannabinoid receptor 1 by exposing mice to synthetic cannabinoids induces a high hypocretin receptor 1 response, then maybe these narcoleptic mice will no longer suffer the narcoleptic symptoms! I could confirm the interaction between these two proteins with a yeast-2 hybrid technique.
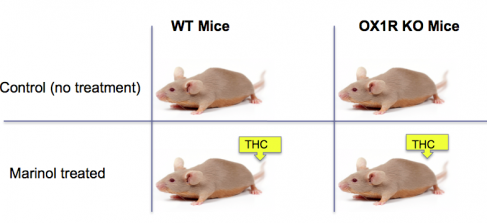
Figure 1: This is an example of an experiment I might conduct to test whether activation of the cannabinoid receptor 1 (using synthetic THC) on mice exhibiting narcoleptic symptoms will activate the hypocretin receptor 1 and treat the narcoleptic symptoms.
Future Directions:
If this interaction between the proteins does function as predicted, then I would like to further investigate this interaction. I would like to determine the smallest level of cannabinoid treatment (and cannabinoid concentration)that would allow the cessation of narcoleptic symptoms. In addition, I would like to determine whether any other side effects result from synthetic cannabinoid treatment. If the side effects outweigh the benefits, then this treatment would not be useful.
I would also like to use tandem affinity purification (TAP) tags to determine any other proteins that might be affected in this pathway. Both of these proteins are located in the lateral hypothalamus. This part of the brain is extremely important in regulating many important body processes critical in maintaining homeostasis including body temperature, hunger, thirst, and circadian rhythms. Therefore, if these hypothalamic receptors do influence eachother which is extremely likely, we should know all we can about these interactions. In the end, after mapping the entire protein interactome found in the hypothalamus, there may be more ways than one to induce the hypocretin receptor 1 response and effectively put an end to the symptoms caused by narcolepsy.
I would also like to use tandem affinity purification (TAP) tags to determine any other proteins that might be affected in this pathway. Both of these proteins are located in the lateral hypothalamus. This part of the brain is extremely important in regulating many important body processes critical in maintaining homeostasis including body temperature, hunger, thirst, and circadian rhythms. Therefore, if these hypothalamic receptors do influence eachother which is extremely likely, we should know all we can about these interactions. In the end, after mapping the entire protein interactome found in the hypothalamus, there may be more ways than one to induce the hypocretin receptor 1 response and effectively put an end to the symptoms caused by narcolepsy.
References:
(1) Chemelli RM et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999 Aug 20;98(4):409-12.
(2) Sandrine Hilairet et al. Hypersensitization of the Orexin 1 Receptor by the CB1 Receptor: EVIDENCE FOR CROSS-TALK BLOCKED BY THE SPECIFIC CB1 ANTAGONIST, SR141716. Epub 2003 Apr 10.
(2) Sandrine Hilairet et al. Hypersensitization of the Orexin 1 Receptor by the CB1 Receptor: EVIDENCE FOR CROSS-TALK BLOCKED BY THE SPECIFIC CB1 ANTAGONIST, SR141716. Epub 2003 Apr 10.
Eric Suchy, Email: [email protected], last updated: May 17, 2010